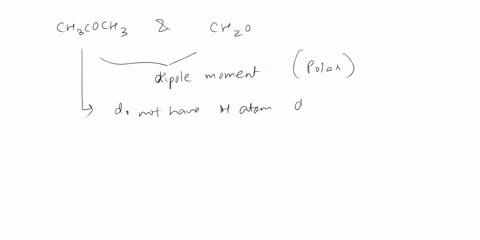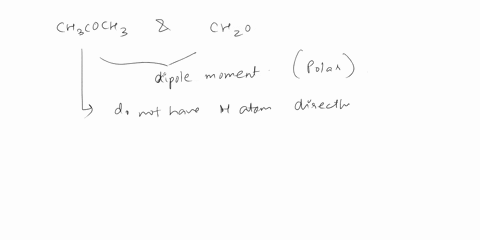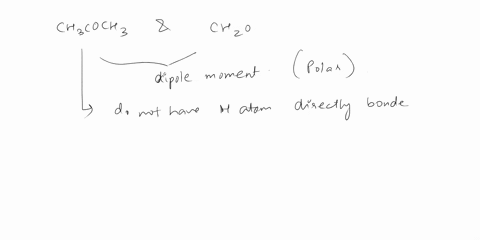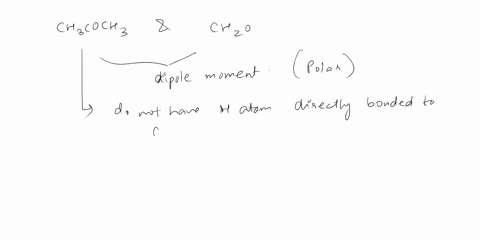
Answer 1 year ago The correct answer is dipole-dipole. The given molecules are acetone with the chemical formula CH3C (O)CH3 and chloroform with the chemical formula CHCl3. They both are considered polar molecules because of the presence of net dipole moment in each molecule.
SOLVED: Give the major force between acetone and chloroform. – dipole-dipole – dispersion – hydrogen bonding – ion-ion – ion-dipole
The major force between acetone and chloroform is known as dipole-dipole interaction or dipole-dipole forces.The answer is dipole-dipole forces. Acetone and chloroform are both polar molecules, meaning they have a partial positive charge on one end and a partial negative charge on the other end.
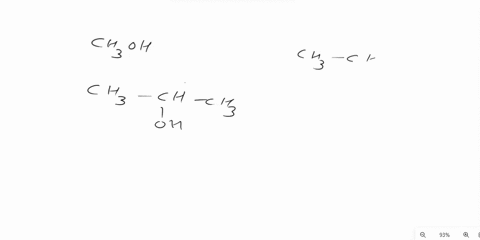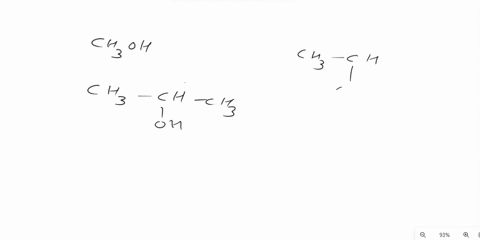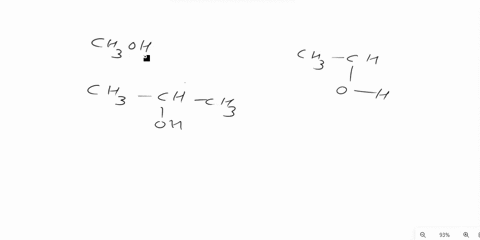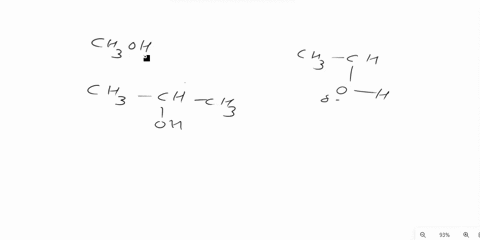
Source Image: numerade.com
Download Image
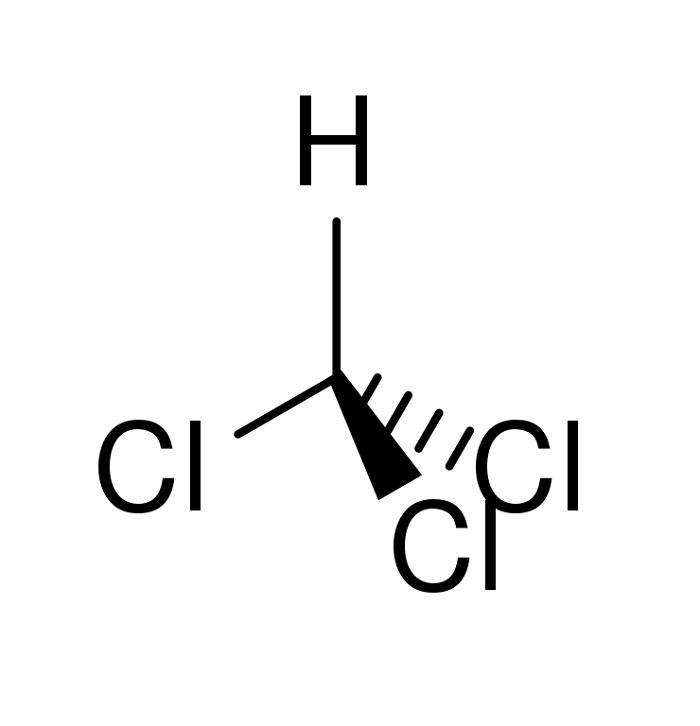
Chemistry Question Give the major force between acetone (CH _3 3 C (O)CH _3 3) and chloroform (CHCl _3 3) A) Dipole-dipole B) Dispersion C) Hydrogen bonding D) Ion-ion E) Ion-dipole Solution Verified Answered 7 months ago Create an account to view solutions Recommended textbook solutions Chemistry: The Molecular Nature of Matter and Change

Source Image: semanticscholar.org
Download Image
Control of the Maximum-Boiling Acetone/Chloroform Azeotropic Distillation System | Industrial & Engineering Chemistry Research Answer link This is very unusual, but I will say hydrogen-bonding. Acetone weakly hydrogen-bonds with chloroform. (When I say weakly, I mean weaker than you would see in typical examples like “NH”_3, “HF”, or “H”_2″O” with themselves.)

Source Image: youtube.com
Download Image
Give The Major Force Between Acetone And Chloroform
Answer link This is very unusual, but I will say hydrogen-bonding. Acetone weakly hydrogen-bonds with chloroform. (When I say weakly, I mean weaker than you would see in typical examples like “NH”_3, “HF”, or “H”_2″O” with themselves.) Section: Chapter Questions Problem 57A See similar textbooks Related questions Question Give the major force between acetone and chloroform. dipole-dipole dispersion hydrogen bonding ion-ion ion-dipole Expert Solution Trending now This is a popular solution! Step by step Solved in 3 steps with 3 images SEE SOLUTION Check out a sample Q&A here
Acetone and chloroform reacts to produce | CLASS 12 | ALDEHYDES AND KETONES | CHEMISTRY | Doubt… – YouTube
Chemistry Chemistry questions and answers Give the major force between acetone and chloroform ion-dipole dipole-dipole hydrogen bonding dispersion ion-ion and can you also please explain why?? This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Chloroform, Exposure Risks, and Government Recommendations
Source Image: onlinesafetytrainer.com
Download Image
When acetone and chloroform are mixed, hydrogen bonding takes place between them. Such a liquid pair cause:(A)Positive deviation from Raoult’s law(B)Negative deviation from Raoult’s law(C)No deviation from raoult’s law(D)Cannot be predicted Chemistry Chemistry questions and answers Give the major force between acetone and chloroform ion-dipole dipole-dipole hydrogen bonding dispersion ion-ion and can you also please explain why?? This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer
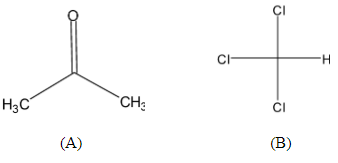
Source Image: vedantu.com
Download Image
SOLVED: Give the major force between acetone and chloroform. – dipole-dipole – dispersion – hydrogen bonding – ion-ion – ion-dipole Answer 1 year ago The correct answer is dipole-dipole. The given molecules are acetone with the chemical formula CH3C (O)CH3 and chloroform with the chemical formula CHCl3. They both are considered polar molecules because of the presence of net dipole moment in each molecule.
Source Image: numerade.com
Download Image
Control of the Maximum-Boiling Acetone/Chloroform Azeotropic Distillation System | Industrial & Engineering Chemistry Research Chemistry Question Give the major force between acetone (CH _3 3 C (O)CH _3 3) and chloroform (CHCl _3 3) A) Dipole-dipole B) Dispersion C) Hydrogen bonding D) Ion-ion E) Ion-dipole Solution Verified Answered 7 months ago Create an account to view solutions Recommended textbook solutions Chemistry: The Molecular Nature of Matter and Change

Source Image: pubs.acs.org
Download Image
The Problem Analysis Of Acetone And Chloroform | PDF Test Match QChat Beta Created by NicoleAlexis49 Terms in this set (22) Give the major force between acetone and chloroform Dipole-dipole Choose the situation that would result in a ∆Hsolution near 0 When |∆H solute| is close to |∆H hydration| Dissolving can be defined as Rate of dissolution>rate of depression Define specific heat capacity

Source Image: slideshare.net
Download Image
Hydrogen-bond dynamics of C-H…O interactions: the chloroform…acetone case. | Semantic Scholar Answer link This is very unusual, but I will say hydrogen-bonding. Acetone weakly hydrogen-bonds with chloroform. (When I say weakly, I mean weaker than you would see in typical examples like “NH”_3, “HF”, or “H”_2″O” with themselves.)

Source Image: semanticscholar.org
Download Image
Why a solution of Chloroform and Acetone shows negative deviation from Raoult’s law? Section: Chapter Questions Problem 57A See similar textbooks Related questions Question Give the major force between acetone and chloroform. dipole-dipole dispersion hydrogen bonding ion-ion ion-dipole Expert Solution Trending now This is a popular solution! Step by step Solved in 3 steps with 3 images SEE SOLUTION Check out a sample Q&A here

Source Image: byjus.com
Download Image
When acetone and chloroform are mixed, hydrogen bonding takes place between them. Such a liquid pair cause:(A)Positive deviation from Raoult’s law(B)Negative deviation from Raoult’s law(C)No deviation from raoult’s law(D)Cannot be predicted
Why a solution of Chloroform and Acetone shows negative deviation from Raoult’s law? The major force between acetone and chloroform is known as dipole-dipole interaction or dipole-dipole forces.The answer is dipole-dipole forces. Acetone and chloroform are both polar molecules, meaning they have a partial positive charge on one end and a partial negative charge on the other end.
Control of the Maximum-Boiling Acetone/Chloroform Azeotropic Distillation System | Industrial & Engineering Chemistry Research Hydrogen-bond dynamics of C-H…O interactions: the chloroform…acetone case. | Semantic Scholar Test Match QChat Beta Created by NicoleAlexis49 Terms in this set (22) Give the major force between acetone and chloroform Dipole-dipole Choose the situation that would result in a ∆Hsolution near 0 When |∆H solute| is close to |∆H hydration| Dissolving can be defined as Rate of dissolution>rate of depression Define specific heat capacity